Hemoccult ICT 2-Day Patient Screening Kit Colorectal Cancer Screening Fecal Occult Blood Test (iFOB or FIT) - 50 Tests
Hemoccult ICT 2-Day Patient Screening Kit Colorectal Cancer Screening Fecal Occult Blood Test (iFOB or FIT). The hemoccult test is a home test that's used to detect the presence of occult blood in your stool. Occult blood is blood in your stool that you can't see in the toilet or on the toilet paper after you have a bowel movement. The hemoccult test is predominantly used as a diagnostic tool for colorectal cancer.
The Hemoccultfi ICT (Immunochemical Test) is a rapid, visually read, qualitative immunochemical chromatographic method for detection of human hemoglobin from blood in fecal samples. Fecal occult blood tests are useful screening aids for detecting primarily lower gastrointestinal (g.i.) disor-ders that may be related to iron deficiency anemia,diverticulitis, ulcerative colitis, polyps, adenomas,colorectal cancers or other g.i. lesions that can bleed.Hemoccultfi ICT is recommended for use by healthprofessionals as part of routine physical examina-tions or when lower g.i. disorders are suspected.
Hemoccult ICT Kit Features
- Specific to human blood
- High sensitivity and specificity
- No dietary or drug restrictions
- Clear, readable results
- Safe, non-invasive
- Easy to use
- CLIA waived
- 50 tests per box
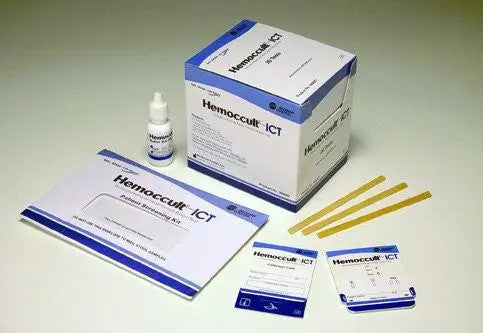
Kit Includes:
- (1) Dispensing Envelope
- (2) Hemoccult ICT Collection Cards
- (2) Flushable Collection Tissue
- (2) Applicator Sticks
- (1) Mailing Pouch
-
Patient Instructions
Uses: Fecal occult blood testing for gastrointestinal disorders.
What makes this FOBT so accurate? The fact that it is specific to whole human hemoglobin and lower GI bleeding. It is not affected by your patients diet, or their medicinal needs, and it is highly sensitive. Hemoccult ICT produces clear, readable results in a safe, non-invasive, easy-to-use way, and produces fewer false positives when compared to traditional guaiac-based FOBTs. Help your patient by using an occult blood test that provides early detection with superior results.
Number: 395261A