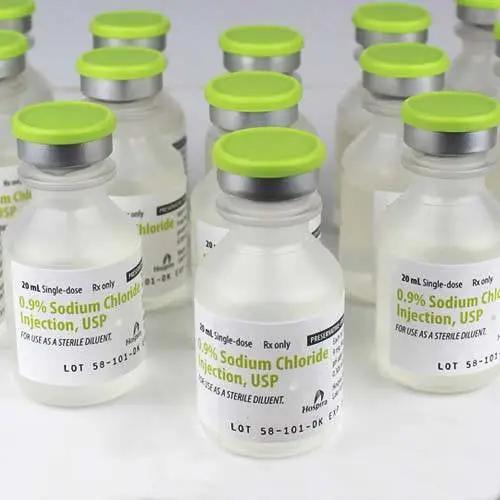
Sodium Chloride 0.9% For Injection 20 mL Vials 25/tray (Rx)
(Note: We don’t Fill Personal Prescriptions)
How to Order:
Sodium Chloride 0.9% For Injection 20 mL Vials are primarily used as a sterile isotonic solution for rehydration, electrolyte balance, and as a vehicle for medication delivery. They facilitate the dilution or dissolution of drugs for intravenous, intramuscular, or subcutaneous injection. This solution closely mimics the body's natural saline concentration, promoting safe and effective medication administration. It is also used in flushing intravenous catheters to maintain patency. Common in hospitals and clinics, it plays a vital role in various therapeutic and hydration needs.
Sodium Chloride 0.9% for Injection is a sterile, non-pyrogenic isotonic solution used primarily as a diluent for reconstituting or diluting medications for injection. This solution does not contain preservatives and is intended for single use. It is supplied by Pfizer Injectables in 20 mL vials, and each tray contains 25 vials.
Details and Features:
Volume: 20 mL per vial
Packaging: 25 vials per tray
Concentration: 0.9% Sodium Chloride (also known as Normal Saline)
Use: Diluent or solvent for medications intended for parenteral administration.
Sterility: Sterile and non-pyrogenic, ensuring safety and efficacy for medical use.
Isotonicity: The solution is isotonic with bodily fluids, which reduces the risk of irritation when mixed with injectable drugs.
Mechanism of Action:
Sodium Chloride 0.9% for Injection serves as an isotonic solution that can safely mix with various medications intended for injection. The sodium chloride maintains osmotic conditions in line with human extracellular fluid, allowing for comfortable administration by helping to prevent irritation or hemolysis (destruction of red blood cells) in infusion therapy.
Warnings:
-
Single Use Only: Does not contain antimicrobial preservatives; any unused portion should be discarded to maintain sterility.
-
Application: Not for direct intravenous administration without the addition of compatible drugs; it is strictly a diluent.
-
Check Compatibility: Always verify compatibility with the drug being mixed as some drugs may precipitate or become inactive.
-
Fluid Balance: Use with caution in patients with diseases causing sodium retention or those on controlled sodium diets.
- Storage Conditions: Store at controlled room temperature and protect from freezing and excessive heat.
Side Effects:
- When used correctly as a diluent, Sodium Chloride 0.9% for Injection is generally safe. However, potential side effects might include:
-
Local Reactions: Site irritation if the solution is not isotonic after mixing or if administered improperly.
-
Imbalance Issues: Large volumes or rapid administration of saline solutions can lead to fluid overload or electrolyte imbalance, particularly if undiluted in those with compromised cardiac or renal function.
- Allergic Reactions: Rare, but hypothetically possible, hypersensitivity reactions to the product’s components.
This solution should only be used under the guidance of a healthcare professional who can ensure it is applied correctly and safely to avoid adverse effects. Proper protocol dictates key checks on drug compatibility and procedural cleanliness when preparing medications with this diluent.